Abstract
Introduction
The optimal re-induction and high dose chemotherapy regimens for transplant-eligible patients with relapsed lymphoma are not known. Our centre has previously established the efficacy and tolerability of single-agent high-dose melphalan and ASCT in patients with relapsed/refractory Hodgkin's lymphoma (HL), and others have similarly demonstrated this with non-Hodgkin's lymphoma (NHL). The activity of alkylating agents such as melphalan is dependent on the extent of DNA damage, and combining melphalan with drugs known to inhibit DNA damage repair would be predicted to produce synergistic effects. Gemcitabine is a nucleoside analogue that has been shown to inhibit DNA damage repair caused by prior exposure to alkylating agents, and has clinical anti-tumour activity against lymphoma, and therefore presents a promising combination with alkylators in high-dose chemotherapy studies for lymphoma.
Objective
To evaluate the safety and efficacy of infusional gemcitabine prior to high-dose melphalan followed by autologous stem cell transplantation in patients with relapsed/refractory lymphoma. The data reported currently reflects a planned interim safety analysis after the first 40 patients enrolled on the study.
Methods
This is an investigator-initiated single centre study currently enrolling patients as of April 2015. Adult patients with relapsed or refractory HL, aggressive NHL, or indolent NHL after at least one prior systemic therapy are eligible for this study. Inclusion/exclusion criteria included ECOG performance status ≤2, no significant end-organ dysfunction, no prior history of stem cell transplantation, no CNS involvement, and chemo-sensitive disease (at least partial remission to salvage chemotherapy) at the time of transplantation. High dose therapy regimen was as follows: On Day -1, IV gemcitabine was administered as a loading bolus of 75 mg/m2, followed by a continuous infusion rate of 10 mg/m2/min, followed immediately by IV melphalan 200 mg/m2. In the phase I portion of the study, the starting dose of gemcitabine was 1.5 g/m2 in the first cohort, with dose escalation based on standard "3+3" design, increasing by 0.5 g/m2 increments at each dose level to maximum of 2.5 g/m2. Dose-limiting toxicity was defined as grade 3 mucositis >5 days, grade 3 skin toxicity >3 days, grade 3 diarrhea >7 days, or any grade 4 non-hematological toxicity.
Results
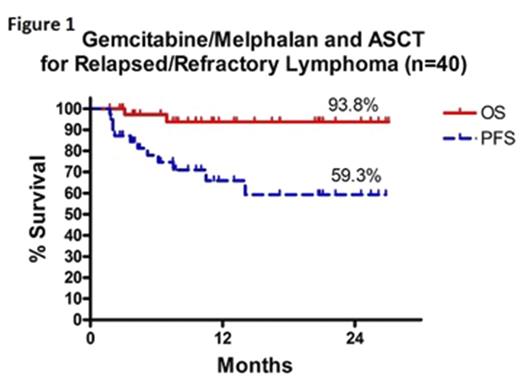
The mean age of the study population was 52 (SD +/-14) with equal numbers of male and female patients. 12 (30%) patients had aggressive NHL (6 DLBCL, 1 PMBCL, 3 ALCL, 2 PTCL), 17 (42.5%) had indolent NHL (15 follicular, 1 lymphoplasmacytic, 1 marginal zone lymphoma), and 11 (27.5%) had HL. At time of relapse, 30% (12/40) of patients had refractory disease after first line therapy, 70% (28/40) had stage III/IV disease, and 6 patients (16%) had >1 prior line of systemic chemotherapy. The median time from relapse to transplant was 90 days (range 12 to 189 days). There were no dose limiting toxicities at 1.5 g/m2 dose of gemcitabine. 8 patients received 2.0 g/m2 and dose-limiting toxicity (grade 3 diarrhea >7 days) was noted in 2 patients, thus the remaining patients received 1.5 g/m2 gemcitabine. 18 patients experienced 24 grade 3-4 adverse non-hematological events: mucositis (7), infectious enterocolitis (5), pneumonia (4), sepsis (2), non-infectious diarrhea (1), esophagitis (1), emesis (1), transaminitis (1), pulmonary edema (1), and stroke (1). There were no treatment-related deaths associated with this regimen. Post-transplant complete remission and partial remission was attained in 25/40 and 9/40 patients, respectively. 6/40 (15%) patients have progressed after transplant, and 2 have died of lymphoma. At a median follow-up of 10 months (range 2-26 months), the progression-free survival is 59.3% and overall survival is 93.8% (Figure 1).
Conclusion
Infusional gemcitabine at a maximum tolerated dose of 1.5 g/m2 in combination with high dose melphalan as a conditioning regimen for autologous stem cell transplantation is tolerable and appears to be efficacious in patients with relapsed/refractory lymphoma.
Stewart: BMS: Honoraria; Abbvie: Honoraria; Janssen: Honoraria; Amgen: Honoraria; Seattle Genetics: Honoraria; Servier: Honoraria; Gilead: Honoraria; Merck: Honoraria; Lundbeck: Honoraria; Roche: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.